Research Interests
The diversity of biological functions expressed by proteins arises from their different three-dimensional structures. Therefore, the key to understanding the catalytic action of these macromolecules is to discover the underlying structure – function relationships that produce a particular activity. To achieve this goal, our laboratory is keenly interested in elucidating the high resolution atomic structures of proteins using X-ray diffraction techniques.
To obtain an even clearer understanding of the mechanistic issues involved in enzymatic activities, we also pursue the study of protein complexes with substrates, inhibitors, transition state analogues and even other proteins. These additional types of studies provide exciting new information on the functioning of proteins that is not accessible by other methods. We also obtain further mechanistic insight into biological function through the combined use of structure-function-mutagenesis analyses. In this way the controlled and systematic replacement of key functional and structural amino acids allows for a truly comprehensive understanding of the roles of individual amino acids. To assist in this process, structural modeling on molecular graphics workstations is used to design mutant proteins with specific properties before they are generated in the laboratory. By combining all the available structural and functional information, our laboratory then seeks to apply this knowledge in the development of novel enzyme inhibitors that have the potential of being human therapeutic drugs.
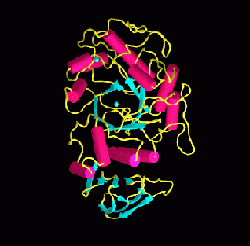
Overall, the current research effort in our laboratory is focused on two proteins. The first involves the critical digestive enzyme human pancreatic alpha-amylase, with the immediate goals of determining the catalytic mechanism of this enzyme and how substrates bind in the active site region next to catalytic residues. As part of this process our laboratory has solved the full three-dimensional atomic structure of human pancreatic alpha-amylase (see drawing below). This structural information, in combination with other functional studies, is being used as the starting point for the design of inhibitors to act as therapeutic drug agents in the treatment of diabetes, obesity and dental caries.
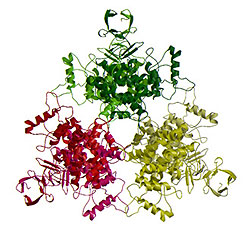
A second system being examined involves a unique hexameric form of the enzyme citrate synthase, which is only found in Gram-negative bacteria. This key metabolic enzyme is allosterically controlled by an unknown mechanism. Our laboratory has recently solved the complete three-dimensional structure of this enzyme (see drawing below) and begun the process of unraveling the structure-function relationships that control its catalytic activity. Given the central role this enzyme plays in the metabolism of Gram-negative pathogens, understanding its mechanism of action could potentially allow for the development of novel anti-microbial compounds.